Recently, Prof. Zhuo Xiong’s research group from Tsinghua University published the latest original research entitled “Expanding Embedded 3D Bioprinting Capability for Engineering Complex Organs with Freeform Vascular Networks” in Advanced Materials, in which they developed a novel bioprinting paradigm by sequential printing in reversible ink template strategy (SPIRIT technique). They successfully printed a ventricle model with a perfusable freeform vascular network by SPIRIT strategy, which is not possible to fabricate using extant embedded 3D printing strategies. The SPIRIT technique offers an unparalleled bioprinting capability to replicate the complex organ geometry and internal structure in a faster manner, which would accelerate the biofabrication and therapeutic applications of tissue and organ constructs.
Creating functional tissues and organs in vitro on demand is a major goal in the field of biofabrication, but the ability to replicate the external geometry of specific organs and their internal structures such as blood vessels simultaneously remains one of the greatest impediments. For the past decade, considerable effort has been made to address this particular challenge. For example, the embedded 3D bioprinting strategy has gained increasing popularity for constructing complex freeform structures; however, it is unable to replicate the internal geometry of native tissues and organs such as the complex blood vessel. Therefore, there is a pressing need to develop new printing paradigm to recapitulate the complexity of both external geometries and internal structures.
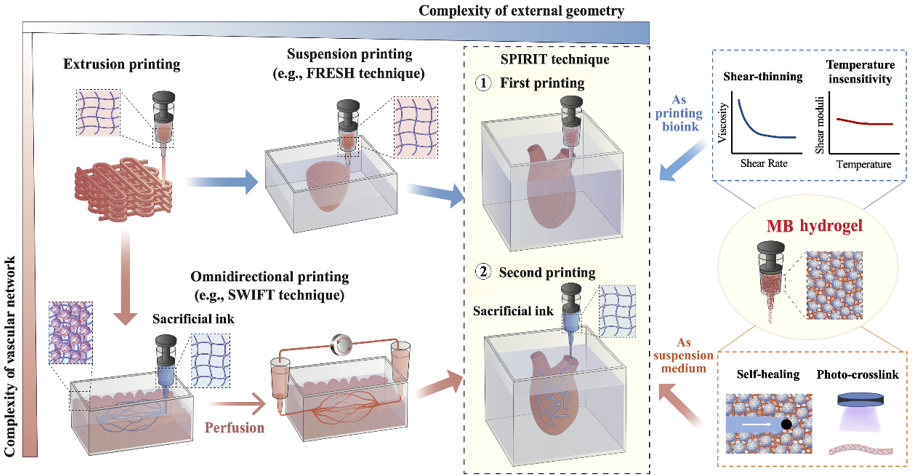
Expanding extrusion bioprinting capability by sequential printing in reversible ink template (SPIRIT) strategy.
Compared with current 3D bioprinting strategies, the SPIRIT technique has the potential to create tissues and organs with extraordinary complexities in both external geometries and internal structures. The SPIRIT technique includes several separate printing processes as follows: i) First printing: we print MB bioink into a Carbopol or gelatin microparticles-based suspension medium to generate complex external structures such as chambered ventricles; ii) Second printing: upon the completion of the first printing process, we print a sacrificial ink such as P-F127 or gelatin into the freshly printed yet uncrosslinked constructs to generate a freeform vascular network by capitalizing the reversible self-recovery behavior of MB bioink; (iii) Crosslinking: we crosslink the printed tissue constructs by in situ photo-crosslinking or incubation at 37℃ while retaining the structural integrity of printed constructs in step (i) and (ii). (iv) Removal of suspension medium and sacrificial ink: We remove the printed constructs from the suspension medium by temperature changing or dilution while the sacrificial ink can be removed simultaneously or separately (Figure 5a).

Perfusable ventricle constructs fabricated by SPIRIT.
The SPIRIT technique highly relies on developing a bioink that can serve as both a bioink and a suspension medium simultaneously. For that purpose, we used the cell-laden microgel-based biphasic (MB) bioink as reported in our previous work (Xiong et al. Adv Funct Mat 2022), which displayed a unique shear-thinning and self-healing over a wide temperature range and a fast in situ photocrosslinking capability. By incorporating MB bioink, the SPIRIT strategy enabled the effective printing of a ventricle model with a perfusable vascular network, which is not possible to fabricate using extant 3D printing strategies. With its flexibility in materials and architectures, the SPIRIT technique provides a new level of capability in the fabrication of complex organs for regenerative applications and complex multi-material constructs for biomedical applications.
Prof. Zhuo Xiong from Tsinghua University is the corresponding author, and Dr. Yongcong Fang from Tsinghua University is the first author. Prof. Wei Sun, Prof. Ting Zhang, and postgraduate students Yihan Guo and Bingyan Wu from Tsinghua University also contributes to the work. This work was funded by the National Natural Science Foundation of China, the new faculty start-up Funding provided by Tsinghua University, the National Key Research and Development Program of China, and the China Postdoctoral Science Foundation.
Link to the paper: https://doi.org/10.1002/adma.202205082
Editor: Li Han

