A team of researchers from the Tsinghua-Berkeley Shenzhen Institute (TBSI) and Shanghai Advanced Research Institute (SARI), Chinese Academy of Sciences, have synthesized a new type of electrocatalyst that is low-cost, durable, and highly efficient for saline water splitting. The discovery was recently published in the journal Advanced Functional Materials.
Water electrolysis is promising for industrial green hydrogen production to achieve a sustainable hydrogen economy, but the high cost of the technology limits its market share. Developing economic catalysts with good efficiency and durability in splitting low-grade water or seawater is important for industrial implementation of the electrolysis to produce green hydrogen, yet is very challenging. Taking hydrogen evolution reaction (HER) as an example, platinum group metals (PGMs) have advantages in performance but suffer the drawbacks of scarcity and high cost. Non-PGM catalysts are cheaper, but have a much poorer performance and few meet industrial demands for use with strong alkalis, saline and low-quality water. Developing economic catalysts that can work well at high current densities in industrial conditions and various extreme electrolytes thus remains a big challenge, preventing the wide use of water splitting for hydrogen production.
Led by TBSI Associate Professor Bilu Liu and SARI Professor Jiong Li, the team has synthesized a new type of electrocatalyst where trace precious metals are strongly anchored on corrosion-resistive matrix. As an example, the produced Pt/Ni-Mo electrocatalyst only needs an overpotential of 113 mV to reach an ultrahigh current density of 2000 mA cm-2 in saline-alkaline electrolyte, standing as the best performance reported so far. It shows high activity and long durability in various electrolytes and under harsh conditions, including strong alkaline and simulated seawater electrolytes, and under elevated temperatures up to 80 oC. This electrocatalyst is produced on a large scale at low cost and shows good performance in a commercial membrane electrode assembly stack, demonstrating its feasibility for practical water electrolysis.
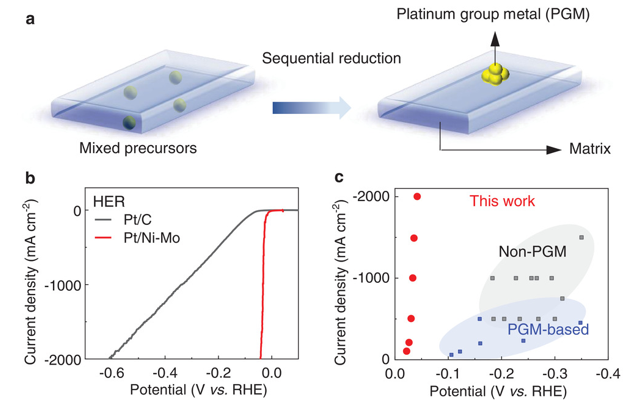
Figure 1. Design principle of the sequential reduction strategy and the high-performance of such catalysts in 1 M KOH solutions.
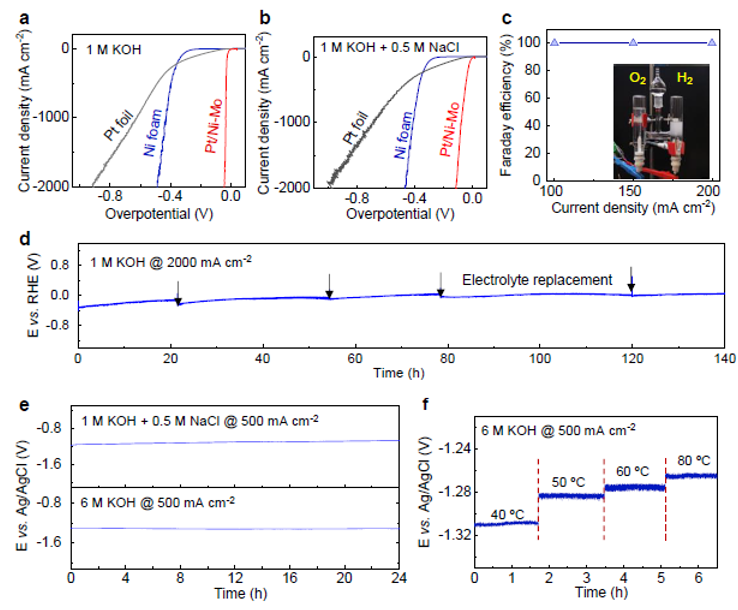
Figure 2. HER performance and durability of Pt/Ni-Mo catalyst at different practical conditions.
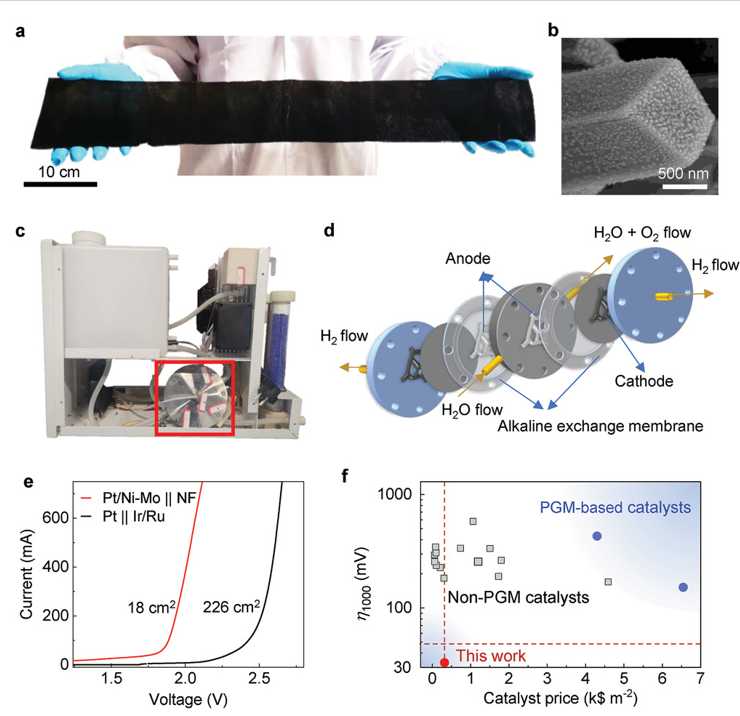
Figure 3. Scaled-up production of Pt/Ni-Mo catalyst and its use in a commercial hydrogen generator.
The work was supported by the National Natural Science Foundation of China, Guangdong Innovative and Entrepreneurial Research Team Program, and the Bureau of Industry and Information Technology of Shenzhen.
Link to full article:
https://onlinelibrary.wiley.com/doi/10.1002/adfm.202010367
Writer: Yang Fengning
Editors: Karen Lee, Li Han

